SEE THE DIFFERENCE
Xiidra works differently from cyclosporine to address dry eye inflammation1-5*

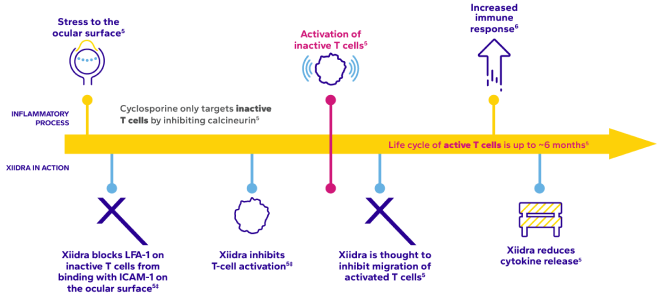
A Key Driver: Inflammation
Up to 65% of patients with dry eye may have clinically significant ocular surface inflammation7
Rapid and
lasting relief
Xiidra can start to deliver symptom relief as early as 2 weeks1‡
ICAM-1, intercellular adhesion molecule 1; LFA-1, lymphocyte function-associated antigen 1.
The exact mechanisms of action of Xiidra and cyclosporine in dry eye disease are not known.
*There are no head-to-head studies of Xiidra and cyclosporine.
†Based on in vitro data.1
‡Xiidra significantly reduced symptoms of eye dryness at 2 weeks (based on Eye Dryness Score compared to vehicle) in 2 of 4 studies, with improvements observed at 6 and 12 weeks in all 4 studies.1
References
- Xiidra. Prescribing information. Bausch & Lomb Inc.
- Restasis. Prescribing information. Allergan; 2017.
- Vevye. Prescribing information. Harrow Eye, LLC; 2023.
- Cequa. Prescribing information. Sun Pharmaceutical Industries Limited; 2022.
- Donnenfeld ED, Perry HD, Nattis AS, Rosenberg ED. Lifitegrast for the treatment of dry eye disease in adults. Expert Opin Pharmacother. 2017;18(14):1517-1524. doi:10.1080/14656566.2017.1372748
- Bron AJ, de Paiva CS, Chauhan SK, et al. TFOS DEWS II pathophysiology report. Ocul Surf. 2017;15(3):438-510.
- Data on file. NPR.0002.USA.24, 2024. Bausch & Lomb Inc.
Indication
Xiidra® (lifitegrast ophthalmic solution) 5% is indicated for the treatment of signs and symptoms of dry eye disease (DED).
Important Safety Information
- Xiidra is contraindicated in patients with known hypersensitivity to lifitegrast or to any of the other ingredients.
- In clinical trials, the most common adverse reactions reported in 5-25% of patients were instillation site irritation, dysgeusia and reduced



