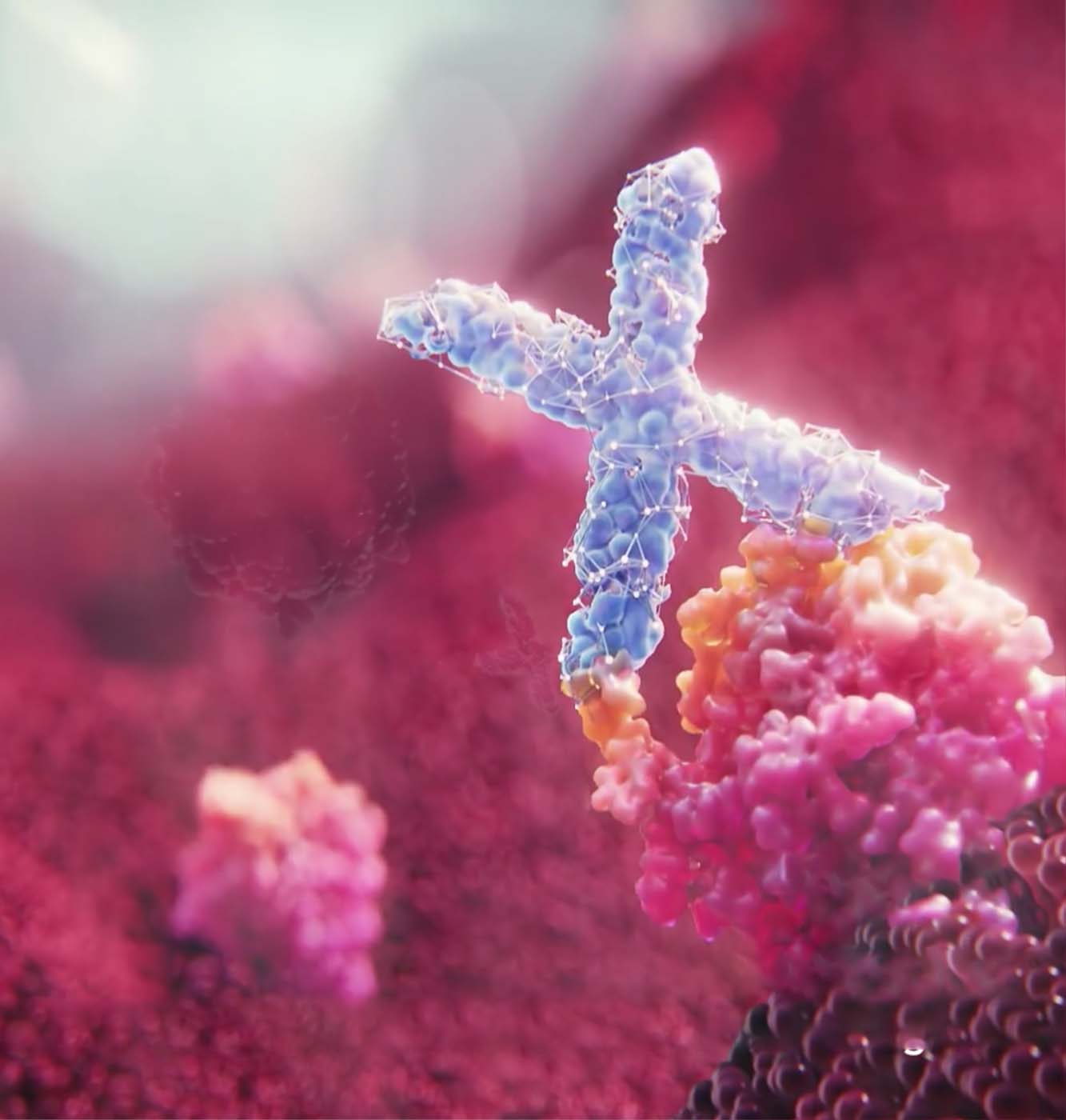
The Xiidra
mechanism of action
Xiidra blocks LFA-1 on T cells from binding with ICAM-1 that may be overexpressed on the ocular surface in dry eye disease and may prevent formation of an immunologic synapse which, based on in vitro studies, may inhibit T cell activation, migration of activated T cells to the ocular surface, and reduce cytokine release.1,3,4
The exact mechanism of action of Xiidra in dry eye disease is not known.1
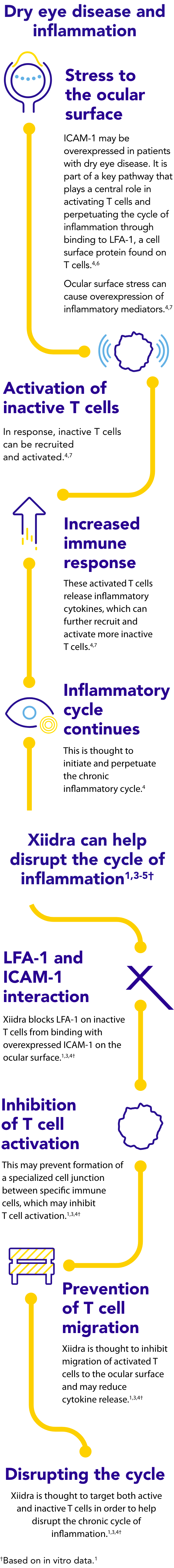
Dry eye disease and inflammation




Xiidra & symptoms of dry eye disease
In Xiidra clinical trials, dry eye disease symptom relief was evaluated.1